Research Article
Impact of Achyranthes aspera Leaf and Stem Extracts on the Survival, Morphology and Behaviour of an Indian Strain of Dengue Vector, Aedes aegypti L. (Diptera: Culicidae) 
2. Acharya Narendra Dev College, University of Delhi, India
 Author
Author  Correspondence author
Correspondence author
Journal of Mosquito Research, 2015, Vol. 5, No. 7 doi: 10.5376/jmr.2015.05.0007
Received: 02 Feb., 2015 Accepted: 18 Apr., 2015 Published: 21 May, 2015
Sharma et al., 2015, Impact of Achyranthes aspera Leaf and Stem Extracts on the Survival, Morphology and Behaviour of an Indian Strain of Dengue Vector, Aedes aegypti L. (Diptera: Culicidae), Journal of Mosquito Research, Vol.5, No.7 1-9 (doi: 10.5376/jmr.2015.05.0007)
Aedes aegypti; well-known vector associated with the transmission of dengue, chikungunya and yellow fever; hasattracted substantial attention worldwide because of the alarming increase in disease statistics. Laboratory investigations were carried out to evaluate the impact of extracts; prepared from the stems and leaves of Achyranthes aspera; on the survival, behaviour and morphology of Ae. aegypti larvae. Healthy and disease-free parts of A. aspera extracted in hexane were screened for their larvicidal activity against early fourth instars of dengue vector in accordance with the WHO protocol. The investigations clearly established the larvicidal efficiency of both the extracts, though hexane stem extracts proved to be 0.82 fold more efficient than the leaf extracts. The bioassay resulted inLC50 value of 68.133 and 82.555 ppm, when larvae were exposed to the hexane stem and leaf extracts of A. aspera, respectively. On the other hand, at LC90 level, the hexane leaves extract proved to be 18% more efficient as compared to the hexane stem extract of A. aspera. Behavioural observations of the treated larvae revealed excitation and restlessness with persistent and aggressive anal biting behaviour indicating the probable effect of the extracts on the neuromuscular system of larvae. Microscopic observations of the dead larvae showed shrunken internal membrane of anal papillae and abnormally stretched body. Further the treated larvae of Ae. aegypti showed distorted alimentary canal with loss of pigmentation and partial or total cell destruction. Our results suggest the probable use of hexane extracts of A. aspera as an efficient and eco-friendly larvicide against Ae. aegypti. Further investigations are needed to identify the bioactive constituent and ascertain its effectiveness in the field conditions.
Aedes aegypti, the chief carrier of Dengue and Chikungunya Virus in India, has engrossed extensive interest at universal level because of rapid spread of these diseases. Since last decade, dengue has emerged as a prime health concern in tropical and sub-tropical expanse of the world; primarily in urban and semi-urban regions; causing increased deaths every year. Since past five decades as high as 30-fold increase in the occurrence of dengue has been observed at the global level. Currently, approximately half of the world’s population has been reported at the risk of dengue fever (WHO, 2015). As per the estimates of World Health Organization, 390 million dengue infections occur each year leading to clinical manifestations of 96 million infections. Though dengue is considered a global concern, yet almost 75% of the world population experiencing dengue resides in the Asia-Pacific region. In India, Ministry of Family and Health Welfare has reported 36486 cases of dengue and 92 fatalities till 31st December, 2014 with highest figures of 7410 cases reported in the state of Maharashtra (NVBDCP, 2015).
Despite of increased prevalence of dengue in many parts of the world, the prevention of the disease primarily lies on the disruption of disease transmission cycle through mosquito control. Since ancient times, the control of mosquito vectors had been mainly dependent on the chemical insecticides-based intervention measures. Nevertheless, recurrent and indiscriminate use of insecticides in the fields has been reported to develop varying amount of insecticide resistance in mosquitoes (Kumar et al., 2002; 2004; Tikar et al., 2008) in addition to which many other concerns; such as toxicity to the non-target organisms, non-degradability causing harm to the environment, and their entry in the food chain has been reported (Borase et al., 2013).
Keeping all the above facts in view, since past few years, researchers have shown renewed interest and are exploring plant extracts and phytochemicals constituents for mosquito management. Several plant extracts have been documented as innocuous substitutes to the synthetic pesticides formulating an ecologically sound and environmentally received mosquito vector and insect pest control program (Warikoo and Kumar, 2013; Mishra et al., 2014). More than 2000 plants species have been recognized as the source of secondary metabolites of use in pest control programs; among which, products of almost 344 species have been reported to possess variable activities against mosquitoes (Sukumar et al., 1991; Ghosh et al., 2012).
Several reports are available in the literature, which reveal the efficacy of various plant parts as larvicides, oviposition deterrents and ovicides against mosquitoes without posing hazards of toxicity to humans (Kumar et al., 2012a,b; Warikoo and Kumar, 2013; 2014). (Promsiri et al., 2006) evaluated the larvicidal activity of extracts prepared from fourteen medicinal plants against Ae. aegypti and revealed 100% larvicidal potential of eight species with LC50 values ranging between 4.1 μg/mL-89.4 μg/mL. Komalamisra et al. (2005) tested the ethanolic extracts prepared from 96 plants against Ae. aegypti larvae and reported only six effective ethanolic extracts prepared from Rhinacanthus nasutus, Derris elliptica, Homalomena aromatica, Trigonostemon redioides, Stemona tuberosa and Acorus calamus. Likewise, the efficacy of ethanolic extracts of dried fruits of three species of peppercorns has been confirmed by (Kumar et al., 2010) against the third and fourth instars of dengue vector. In 2012, Kumar et al. (a) screened fifteen local plants against dengue larvae and reported the hexane leaf extract of Lantana camara as the most effective larvicide with LC50 value of 30.71 ppm They also reported the significant larvicidal potential of the extracts made from different parts of other five plants; Achyranthes aspera, Zingiber officinalis, Ricinus communis, Trachyspermum ammi and Cassia occidentalis with LC50 values ranging from 55.0 to 74.67 ppm.
Achyranthes aspera L. (Amaranthaceae), commonly called devil's horsewhip is an herb native to Africa and Asia. The seeds and leaves of the plant are consumed by human beings and are a part of religious ceremonies in India (Ragupathy and Newmaster, 2009). The extracts prepared from the leaves are also reported to possess prothyroidic and antiperoxidative activity in rats (Tahiliani and Kar, 2000). It has also been reported that saponins isolated from the ethyl acetate extract of A. aspera leaves act as potential larvicides against Ae. aegypti and Culex quinquefasciatus Say while the extracts prepared from the leaves, flowers, and the seeds of A. aspera exhibited biological activity against the 4th instars of Anopheles subpictus Grassi and Cx. tritaeniorhynchus Giles larvae (Bagavan et al., 2008).
Keeping the anti-mosquito potential of A. aspera in view, the leaves and stems of locally available plants were collected from New Delhi, India and extracted in hexane. The impact of these extracts was investigated on the survival, morphology and behaviour of the early fourth instars of dengue vector, Ae. aegypti. The results of the present investigations may not only be helpful to promote mosquito management research aiming at the formulation of new eco-friendly substitute for chemical insecticides but also help in controlling of this undesirable weed.
1 Materials and Methods
1.1 Rearing of Aedes aegypti
The colony of the dengue fever mosquito; Ae. aegypti; was maintained in an insectary under controlled conditions of 28 ± 1℃, 80 ± 5% RH, and 14/10 L/D photoperiod (Warikoo and Kumar, 2013). Adult mosquitoes were reared in cloth cages. A small swab of moist cotton was placed on the top of each cage to provide water to adult mosquitoes. A glass Petri plate containing 3-4 water-soaked deseeded split raisins was placed in the cage as a source of the food, primarily for the male adults. Periodic blood meals required for the egg maturation were provided to the female adults after two days of their emergence. The eggs were collected in an enamel bowl (6 cm diameter) lined with Whatman filter paper and filled half with de-chlorinated tap water. The filter strips and water laid with eggs were transferred to a tray (25 cm × 30 cm × 5 cm) containing de‑chlorinated tap water for hatching. The hatched larvae were provided daily with food containing finely grounded dog biscuits and live yeast powder (3:1 w/w). Water was changed every alternate day in order to prevent formation of scum on the surface of water. The pupae formed were separated into an enamel bowl and kept placed inside the cloth cages for adult emergence.
1.2 Collection of Achyranthes aspera
The plant A. aspera, selected for the present investigations, was collected from the surrounding parts of Acharya Narendra Dev College and brought to the laboratory in sterile polythene bags. The collected plants were carefully washed with tap water and cleared of dust or any undesirable particles wedged to them. Careful observations were made to separate the plants with any kind of disease or infection and were discarded, if found any. Healthy leaves and stems of the selected plant were separated and dried for about 20 days at room temperature of 27±2˚C till they dried completely taking care of fungal infections.
1.3 Preparation of A. aspera Extract
The dried leaves and stems of A. aspera were mechanically grinded, separately and sieved carefully to obtain fine powder. The dry powders of plant parts were weighed and 250 g of each dried and powdered part was soaked in 1000 mL of hexane. The soaked parts were left undisturbed for 5 to 7 days and the hexane extracts, thus obtained, were concentrated using a vacuum evaporator (Buchi Type) at a temperature not exceeding their boiling temperature of 60℃. After complete evaporation of the solvent, the concentrated extract was stored in a refrigerator at 4℃.
1.4 Impact of A. aspera extract on the survival of Ae. aegypti larvae
The larvicidal bioassay was performed against the early fourth instar larvae of Ae. aegypti at 28±1℃ in accordance with the procedure described by WHO with slight modifications (WHO, 2005). The graded series of the extracts was prepared using ethanol as the solvent. Batches of 20 early fourth instars of Ae. aegypti taken in plastic bowls containing 99 ml of distilled water were transferred to glass jar containing 100 mL of distilled water and 1 mL of a particular concentration of A. aspera extract. Three replicates were carried out simultaneously for each extract making a total of 60 larvae for each test. Controls were exposed to the solvent, i.e., ethanol alone.
The dead and moribund larvae were recorded after 24 h by touching gently with the help of a glass rod. The larvae without any sign of movements were considered dead while were considered moribund if they moved a little but did not show any kind of vigorous and active movement. The tests with more than 20% mortality in controls or with more than 20% pupae formed were discarded and repeated again. The control mortality ranged between 5 and 20%, if any, was corrected using Abbott’s formula (Abbott, 1925).
The data was subjected to regression analysis using computerized SPSS 22.0 Program. The LC30, LC50 and LC90 values with 95% fiducial limits, chi-square and regression coefficient were calculated in each bioassay to assess the significance and measure difference between the test samples.
1.5 Impact of A. aspera extract on the behaviour of Ae. aegypti larvae
During the larvicidal bioassay with A. aspera extracts, the larvae of Ae. aegypti were monitored carefully for behavioural modifications. The observations included wriggling speed, movements in different directions, aggregation behaviour etc. The larval behaviour was recorded and photographed with Canon Power Shot SX50HS. Similar observations were made in control larvae for comparison with the treated larvae.
1.6 Impact of A. aspera extract on the morphology of Ae. aegypti larvae
The early fourth instars of Ae. aegypti were taken in glass jars, each containing 199 mL of water and 1 mL of A. aspera leaves and stem hexane extracts at LC30, LC50 or LC90. The larvae found dead after 24 h were separated and studied under light microscopy for morphological aberrations. Larvae were scrutinized after mounting with Hoyer’s medium and morphological changes in body segments including the head, thorax, and abdomen, and other organs such as the eyes, antennae, setae, and anal gills were observed, photographed and compared with those of the controls.
2 Results
The results of bioassay performed with the hexane leaves and stem extracts of A. aspera against early fourth instars of Ae. aegypti are presented in Table 1. Our investigations clearly established the larvicidal efficiency of both the extracts exhibiting LC50 values below 100 ppm, though hexane stem extracts proved to be 0.82 fold more efficient than the leaf extracts. The 24 h larvicidal bioassay with hexane stem extract of A. aspera resulted in LC50 value of 68.133 ppm while the value of 82.555 ppm was obtained when early fourth instars of Ae. aegypti were treated with the leaf extracts. Likewise, the larvicidal bioassay with hexane leaves extract also resulted in 18% lower LC90 value of 139.8 ppm as compared to LC90 value of 115.0 ppm resulted when the larvae of Ae. aegypti were assayed with hexane stem extract of A. aspera (Table 1).
|
Table 1 Larvicidal activity of the leaf and stem hexane extracts of Achyranthes aspera against early fourth instars of Aedes aegypti |
When the early fourth instars of Ae. aegypti treated with A. aspera stem and leaves extracts were carefully observed for their behaviour, the larvae showed a peculiar pattern. Immediately after exposure to the extracts, all larvae exhibited natural and vigorous movements (Figure 1A). However, only 2 min of exposure resulted in agitation and restlessness in the dengue larvae (Table 2). Gradually, the larvae resorted to swift wriggling movements which persisted for approximately 10 to 15 min following which 15 to 61.6% larvae started sinking at the bottom of glass jars. In contrast, remaining larvae exhibited severe restlessness behaviour with aggressive self-biting of their anal papillae with their mouth parts (Figure 1B and 1C). Consequently, the larvae formed ring-like structures and showed coiling movements. After 30 min, 81.6 to 86.6% larvae, exposed to LC90 of extracts, showed tremor and convulsions followed by paralysis and sank down to bottom. On the other hand, at lower concentrations, the larvae exhibited increasingly aggressive behaviour. After 1 h of exposure 11.6 to 76.6% larval mortality was recorded in those exposed to the leaf plant extracts whereas exposure to stem plant extract led to only moribund stage. Interestingly, after 2 h, all larvae exposed to stem extract of A. aspera were dead whereas exposure to leaf extract could cause 100% mortality after 3 h of exposure, indicating the higher toxicity potential of stem extract. Gradually, larval mortality was observed in each jar, which increased with the rise in concentration.
 Table 2 Impact of the leaf and stem hexane extracts of Achyranthes aspera on the behaviour of early fourth instars of Aedes aegypti |
|
Figure 1 Digital photomicrographs of early IV instar larvae of Ae. Aegypti |
The microscopic observations of the morphological features of the control and treated larvae of Ae. aegypti revealed that larvae did not exhibit any aberrations in the organs such as eyes, antennae, mouth, setae, siphon and ventral brushes. However, the anal papillae of all the treated larvae showed abnormal pigmentation and shrinkage in their internal structure, the morphological aberrations increasing with the increased concentration whereas the external features of papillae were found to be normal as observed in the control larvae (Figure 2, 3 and 4). The investigations also revealed that the larvae treated with stem extract of A. aspera exhibited more severe aberrations in anal papillae as compared to the larvae treated with leaf extract.
 Figure 2 Light photomicrograph of the anal gills of untreated early IV instars of Aedes aegypti |
|
Figure 3 Light photomicrographs of the anal gills of early IV instar larvae of Aedes aegypti treated with hexane stem extract of Achyranthes aspera treated at (A) LC30 level; (B) LC50 level and (C) LC90 level |
|
Figure 4 Light photomicrographs of the anal gills of early IV instar larvae of Aedes aegypti treated with hexane leaf extract of Achyranthes aspera treated at (A) LC30 level; (B) LC50 level and (C) LC90 level |
Another remarkable observation was the abnormal stretched shape of the larval body with peculiar pigmentation as compared to that observed in control larvae (Figure 5, 6), the pigmentation being more pronounced in the larvae treated with hexane stem extract of A. aspera. Further the treated larvae of Ae. aegypti also showed distorted alimentary canal with loss of pigmentation and partial or total cell destruction. On the contrary, the effect on alimentary canal was more visible in the larvae treated with hexane leaves extract of A. aspera (Figure 6).
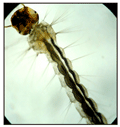 Figure 5 Light photomicrograph of the alimentary canal of control early IV instar of Aedes aegypti |
|
Figure 6 Light photomicrograph of the alimentary canal of early IV instars of Aedes aegypti treated with (A) Hexane stem and (B) Hexane leaf extract of Achyranthes aspera |
3 Discussion
The extensive use of synthetic chemical insecticides has resulted in environmental degradation, hazards and resistance to these insecticides in major vector species. This has necessitated the formulation of a more potent and environment-friendly insecticide. Since last few years, the researchers have focused their interest towards the control of mosquito larvae with the use of plant extracts. Plants have been known to produce various chemicals, many of which have reported to possess medicinal, insecticidal and pesticidal properties. More than 2000 plant species have been known to produce chemical factors and metabolites of value in pest control programmes. A number of plant extracts has been reported to possess larvicidal, adulticidal or repellent activities against different species of mosquitoes (Ghosh et al., 2012).
The extracts prepared from the leaves of A. aspera are reported to possess prothyroidic and anti-peroxidative activity in rats (Tahiliani and Kar, 2000). The extracts prepared from the leaves, flowers, and the seeds of A. aspera have been reported to exhibit bioefficacy against the fourth instars of An. subpictus and Cx. tritaeniorhynchus whereas the saponins isolated from the ethyl acetate extract of A. aspera leaves have been found as potential larvicides against Ae. aegypti and Cx. quinquefasciatus (Bagavan et al., 2008). Present investigations clearly revealed the efficacy of hexane extracts prepared from the leaves and stem of A. aspera against early IV instars of Ae. aegypti. Bhattachrya and Chandra (2013) assayed different instars of Cx. vishnui with acetone leaf extracts of A. aspera and reported LC50 values ranging from 35.46 ppm to 63.39 ppm, the value decreasing with the increase in the time of exposure. The larvicidal efficacy of essential oils of A. aspera has also been reported by (Khandagle et al., 2011) against the early fourth instars of Ae. aegypti with the LC50 and LC90 values of 761 ppm and 817 ppm, respectively. Earlier, (Kumar et al., 2012a) revealed the appreciable larvicidal potential of hexane extracts prepared from different plant species against Ae. aegypti. They reported LC50 values ranging from 30.00 to 74.67 ppm when the larvae were exposed to the extracts made from different parts of A. aspera, Cassia occidentalis, Lantana camara, Ricinus communis, Trachispermum ammi and Zingiber officinalis. (Kumar et al., 2012b) also tested different parts of a weed, Parthenium hysterophorus, extracted in different solvents against Ae. aegypti larvae and found hexane and petroleum ether extracts being the most effective resulting in 100 % mortality.
Larvicidal bioassays conducted by Warikoo and Kumar (2013) with hexane root extracts of Argemone mexicana has resulted in the LC50 and LC90 values of 91.331 ppm and 156.684 ppm, respectively against early fourth instars of Ae. aegypti when exposed for 24 h. They also reported 1.1-fold rise in the cidal potential of the extract when the larvae were exposed for 48 h. In 2007, Raj Mohan and Ramaswamy reported the LC50 value of 256.70 and 227.20 ppm, respectively when the early IV instars of Ae. aegypti and Cx. quinquefasciatus were exposed to the Aegeratina adenophora leaf extracts for 24 h. The investigations with the hexane extracts prepared from the dried fruits of three species of peppercorns; Long pepper, Piper longum L., Black pepper, P. nigrum, and White pepper, P. nigrum revealed the Black P. nigrum extract as the most effectual larvicide against larvae of Ae. aegypti followed by that of P. longum, whereas White pepper was found as the least effective (Kumar et al., 2010). In contrast, the hexane extracts prepared from the leaves of Citrus sinensis showed reasonable larvicidal potential against dengue vector, the bioassay resulting in very high LC50 and LC90 values of 446.84 and 1370.96 ppm, respectively (Warikoo et al., 2012).
The behavioural observations of the early IV instar larvae of Ae. aegypti treated with the leaf and stem extracts of A. aspera revealed excitation, restlessness, and aggressive movements in the larvae. These symptoms being similar to those caused by nerve poisons suggested that the extracts of A. aspera could possibly act as cytolysin and had an impact on the neuromuscular system of larvae. Our findings corresponded to those of a few earlier studies (Choochote et al., 2004; 2005; Dharmagadda et al., 2005; Chaithong et al., 2006; Kumar et al., 2010) suggesting the extracts acting as nerve poisons; though, the aggressive and uncoordinated movements along with other toxic symptoms were observed at relatively different time intervals. Our studies also showed coiling movements in the treated larvae with aggressive anal biting behaviour further enforcing the neurotoxic impact of the extracts on Ae. aegypti larvae. Similar behavioural observations have been reported by Warikoo and Kumar (2013) in the larvae of Ae. aegypti when assayed with extracts prepared from A. mexicana. It has been found that the anal papillae of mosquitoes have some role in regulating electrolyte balance required for the life sustainability (Becker et al., 2010). This suggests the cytotoxic effects of A. aspera extract leading to electrolyte imbalance in the anal region causing violent anal biting.
Present investigations also resulted in the structural aberrations in the anal papillae of early IV instars of Ae. aegypti when exposed to stem and leaf extracts of A. aspera. The microscopic observations of the anal papillae showed shrinkage in the internal membrane of the anal papillae, while the external membranes did not show any modifications. These results are in agreement with the similar internal shrinkage reported by Warikoo and Kumar (2013) in the anal papillae of Ae. aegypti larvae when assayed with variable extracts of A. mexicana. Likewise destructed anal papillae with shrunken cuticular border have been observed by (Insun et al., 1999) in Cx. quinquefasciatus larvae when treated with ethanolic extract of Kaempferia galangal. It has been suggested that the structural deformity in the anal papillae possibly led to anomalous functions which may have led to the disturbance in osmotic and ionic regulation (Chaithong et al., 2006). They also suggested that the disruptive regulatory functions may have caused the mortality of the Ae. aegypti larvae. In the present investigations, the larvae of Ae. aegypti exposed to A. aspera extracts also showed distorted midgut with loss of pigmentation and partial or total cell destruction. Similar observations in the midgut of Ae. aegypti were reported by (Chaithong et al., 2006) when the larvae were exposed to pepper extracts.
Our investigations have established the potential of A. aspera extracts against Ae. aegypti larvae and their possible use as the potential larvicides against mosquito control. However, the range of types and levels of active constituents in these extracts may be responsible for the variability in their potential against Ae. aegypti. Further investigations are needed to understand the mechanism involved leading to larval mortality, to explore and characterize the bioactive constituents involved, and to identify their mode of action.
Author’s Contributions
AS conceived the idea, conducted the experiments and wrote the manuscript. AS and PT designed the experiments and sourced for funds. PT and SK supervised and guided the experiments. AS and SK recorded the microscopic observations and SK helped in the statistical analysis of the results. All authors were involved in the preparation of manuscript.
Competing interest
The authors declare that they have no competing interest.
Acknowledgements
Authors are highly thankful to Dr. Savithri Singh, Principal, Acharya Narendra Dev College, University of Delhi for providing infrastructure and research facilities.
References
Abbott W.B., 1925, A method for computing the effectiveness of an insecticide. Journal of Economic Entomology, 18, 265–267
http://dx.doi.org/10.1093/jee/18.2.265a
Bagavan A., Rahuman A.A., Kamaraj C., and Geetha K., 2008, Larvicidal activity of saponin from Achyranthes aspera against Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Parasitology Research, 103(1): 223-229
http://dx.doi.org/10.1007/s00436-008-0962-z
Becker N., Petric D., Zqomba M., Boase C., Madon M., Dahl C., and Kaiser A., 2010, Mosquitoes and their control, 2nd edn. Springer, New York, pp: 409–599
http://dx.doi.org/10.1007/978-3-540-92874-4
Bhattacharya K., and Chandra G., 2013, Bioactivity of Achyranthes aspera (Amaranthaceae) foliage against the Japanese encephalitis vector Culex vishnui Group. Journal of Mosquito Research, 3(13) 89-96
http://dx.doi.org/10.5376/jmr.2013.03.0013
Borase H.P., Patil C.D., Salunkhe R.B., Narkhede C.P., Salunke B.K. and Patil S.V. 2013, Phyto-synthesized silver nanoparticles: A potent mosquito biolarvicidal agent. Journal of Nanomedicine and Biotherapeutic Discovery, 3:1-7
http://dx.doi.org/10.4172/2155-983X.1000111
Chaithong U., Choochote W., Kamsuk K., Jitpakdi A., Tippawangkosol P., Chaiyasit D., Champakaew D., Tuetun B., and Pitasawat B., 2006, Larvicidal effect of pepper plants on Aedes aegypti (L.) (Diptera: Culicidae). Journal of Vector Ecology, 31:138-144
http://dx.doi.org/10.3376/1081-1710(2006)31[138:LEOPPO]2.0.CO;2
Choochote W., Chaiyasit D., Kanjanapothi D., Rattanachanpichai E., Jitpakdi A., Tuetun B., and Pitasawat B., 2005, Chemical composition and antimosquito potential of rhizome extract and volatile oil derived from Curcuma aromatica against Aedes aegypti (Diptera: Culicidae). Journal of Vector Ecology, 30:302–309
http://dx.doi.org/10.1002/ps.1693
Choochote W., Tuetun B., Kanjanapothi D., Rattanachanpichai E., Chaithong U., Chaiwong P., Jitpakdi A., Tippawangkosol P., Riyong D., and Pitasawat B., 2004, Potential of crude seed extract of celery, Apium graveolens L., against the mosquito Aedes aegypti (L.) (Diptera: Culicidae). Journal of Vector Ecology, 29(2): 340-346
Dharmagadda V.S.S., Naik S.N., Mittal P.K., and Vasudevan P., 2005, Larvicidal activity of Tagetes patula essential oil against three mosquito species. Bioresource Technology, 96:1235–1240
http://dx.doi.org/10.1016/j.biortech.2004.10.020
Ghosh A., Chowdhury N., and Chandra G., 2012, Plant extracts as potential mosquito larvicides. Indian Journal of Medical Research, 135: 581-598
Insun D., Choochote W., Jitpakdi A., Chaithong U., Tippawangkosol P., and Pitasawat B., 1999, Possible site of action of Kaempferia galangal in killing Culex quinquefasciatus larvae. Southeast Asian Journal of Tropical Medicine and Public Health, 30: 195-199
Isman, M.B., 2000, Plant essential oils for pest and disease management. Crop Protection, 19, 603-608
http://dx.doi.org/10.1016/S0261-2194(00)00079-X
Khandagle A.J., Tare V.S., Raut K.D., and Morey, R.A., 2011, Bioactivity of essential oils of Zingiber officinalis and Achyranthes aspera against mosquitoes. Parasitology Research, 109: 339-343
http://dx.doi.org/10.1007/s00436-011-2261-3
Komalamisra, N., Trongotokit, Y., Rongsriyam, Y., and Apiwathnasorn, C., 2005, Screening for larvicidal activity in some Thai plants against four mosquito vector species. Southeast Asian Journal of Tropical Medicine and Public Health, 36(6):1412-1422
Kumar S., Thomas A., Sahgal A., Verma A., Samuel T., and Pillai, M. K. K., 2002, Effect of the synergist, Piperonyl Butoxide, on the development of deltamethrin resistance in yellow fever mosquito, Aedes aegypti L. (Diptera: Culicidae). Archives of Insect Biochemistry and Physiology, 50: 1-8
http://dx.doi.org/10.1002/arch.10021
Kumar S., Thomas A., Sahgal A., Verma A., Samuel T., and Pillai, M. K. K., 2004, Variations in the insecticide resistance spectrum of Anopheles stephensi Liston on selections with deltamethrin and deltamethrin/PBO combinations. Annals of Tropical Medicine and Parasitology, 98: 861-871
Kumar S., Warikoo R., and Wahab N, 2010, Larvicidal potential of ethanolic extracts of dried fruits of three species of peppercorns against different instars of an Indian strain of dengue fever mosquito, Aedes aegypti L. (Diptera: Culicidae). Parasitology Research, 107: 901-907
http://dx.doi.org/10.1007/s00436-010-1948-1
Kumar S., Wahab N., Mishra M. and Warikoo R., 2012a, Evaluation of 15 local plant species as larvicidal agents against an Indian strain of dengue fever mosquito, Aedes aegypti L. (Diptera: Culicidae). Frontiers in Physiology, 3(104): 1-6
http://dx.doi.org/10.3389/fphys.2012.00104
Kumar S., Nair G., Singh A.P., Batra S., Wahab N., and Warikoo R., 2012b, Evaluation of the larvicidal efficiency of stem, roots and leaves of the weed, Parthenium hysterophorus (Family: Asteraceae) against Aedes aegypti L. Asian Pacific Journal of Tropical Diseases, 2: 395-400
Liu N., Xu Q., Zhu F., and Zhang L., 2006, Pyrethroid resistance in mosquitoes. Insect Science, 13: 159–166
Mishra M., Gupta K.K., and Kumar S., 2014, Impact of stem extracts of yellow oleander, Thevetia neriifolia (Apocynaceae) on the feeding potential of IV Instars of gram pod borer, Helicoverpa armigera Hübner (Lepidoptera: Noctuidae). International Journal of Advanced Life Sciences, 7: 408-416
National Vector Borne Disease Control Programme (NVBDCP), 2015, Dengue Cases and Deaths in the Country since 2007. Available at: http://nvbdcp.gov.in/den-cd.html (Accessed on February 16, 2015)
Promsiri S., Naksathi A., Kruatrachue M., and Thavara, U., 2006, Evaluations of larvicidal activity of medicinal plant extracts to Aedes aegypti (Diptera: Culicidae) and other effects on a non-target fish. Journal of American Mosquito Control Association, 22: 292-295
Ragupathy S., and Newmaster S.G., 2009, Valorizing the ‘Irulas’ traditional knowledge of medicinal Plants in the Kodiakkarai Reserve Forest, India. Journal of Ethnobiology and Ethnomedicine, 5:10-22
http://dx.doi.org/10.1186/1746-4269-5-10
Raj Mohan D., and Ramaswamy M., 2007, Evaluation of larvicidal activity of the leaf extract of a weed plant, Ageratina adenophora, against two important species of mosquitoes, Aedes aegypti and Culex quinquefasciatus. African Journal of Biotechnology, 6: 631-638
Remia K.M., and Logaswamy S., 2010, Larvicidal efficacy of leaf extract of two botanicals against the mosquito vector Aedes aegypti (Diptera: Culicidae). Indian Journal of Natural Products and Resources, 1(2): 208-212
Sakthivadivel M., and Thilagavathy D., 2003, Larvicidal and chemosterilant activity of the acetone fraction of petroleum ether extract from Argemone mexicana L. seed. Bioresource Technology, 89: 213-216
Sukumar K., Perich M.J., and Boombar L.R., 1991, Botanical derivatives in mosquito control: a review. Journal of American Mosquito Control Association, 7: 210-237
Tahiliani P., and Kar A., 2000, Achyranthes aspera elevates thyroid hormone levels and decreases hepatic lipid peroxidation in male rats. Journal of Ethnopharmacology, 71: 527-532
http://dx.doi.org/10.1016/S0378-8741(00)00170-7
Tikar S.N., Mendki M.J., Chandel K., Parashar B.D., and Prakash S., 2008, Susceptibility of immature stages of Aedes (Stegomyia) aegypti; vector of dengue and chikungunya to insecticides from India. Parasitology Research, 102: 907-913
http://dx.doi.org/10.1007/s00436-007-0848-5
Warikoo, R., Ray, A., Sandhu, J.K., Samal, R., Wahab, N., and Kumar, S, 2012, Larvicidal and irritant activities of hexane leaf extracts of Citrus sinensis against dengue vector Aedes aegypti L. Asian Pacific Journal of Tropical Biomedicine, 2: 152-155
http://dx.doi.org/10.1016/S2221-1691(11)60211-6
Warikoo R., and Kumar S., 2013, Impact of Argemone mexicana extracts on the cidal, morphological, and behavioural response of dengue vector, Aedes aegypti L. (Diptera: Culicidae). Parasitology Research, 112(10): 3477-3484
http://dx.doi.org/10.1007/s00436-013-3528-7
Warikoo R., and Kumar S., 2014, Oviposition altering and ovicidal efficacy of root extracts of Argemone mexicana against dengue vector, Aedes aegypti (Diptera: Culicidae). Journal of Entomology and Zoology Studies, 2 (4): 11-17
WHO Report, 2005, World Malaria Report. Geneva: WHO/UNICEF.
World Health Organization (WHO), 2015, Dengue and severe dengue. http://www.who.int/mediacentre/factsheets/fs117/en/. (Accessed February 15, 2015)
. PDF(443KB)
. HTML
Associated material
. Readers' comments
Other articles by authors
. Aarti Sharma
. Sarita Kumar
. Pushplata Tripathi
Related articles
. Aedes aegypti
. Achyranthes aspera
. Larvicide
. Behaviour
. Morphology
Tools
. Email to a friend
. Post a comment